- enantiomerism
- In chemistry, isomerism in which the molecules in their configuration are related to one another like an object and its mirror image (enantiomers) and, consequently, are not superimposable; e. entails optical activity, both enantiomers (in identical amounts) rotating the plane of polarized light equally, but in opposite directions.
* * *
en·an·ti·om·er·ism (en-an″te-omґər-iz-əm) [Gr. enantios opposite + mero- + -ism] the relationship between two stereoisomers having molecules that are mirror images of each other. Enantiomers have identical chemical and physical properties in an achiral environment. However, they form different products when reacted with other chiral molecules, and they exhibit optical activity. The enantiomer that rotates the plane of polarization of a beam of polarized light in the clockwise direction is indicated by the prefix (+)-, formerly d- or dextro-. The other enantiomer rotates the plane of polarization an equal amount in the counterclockwise direction and is indicated by the prefix (−)-, formerly l- or levo-. Two conventions are used to designate the actual configurations of enantiomers. The D, L system (see D-) is used to denote the configuration of carbohydrates relative to D-(+)-glyceraldehyde and of amino acids relative to L-(−)-serine. The R,S system (see R-) is a more general system used to specify the absolute configuration at every asymmetric carbon atom. An equimolar mixture of enantiomers (a racemic form or racemic modification) is optically inactive and is designated by the prefixes (±)-, DL-, or dl-.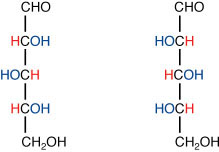
 Enantiomerism.
Enantiomerism.
Medical dictionary. 2011.
